Introduction: Clonal hematopoiesis of indeterminate potential (CHIP) is a common age-related condition arising from somatic mutations in hematopoietic stem cells that leads to mutant cell clonal progeny in the blood (variant allele frequency [VAF] > 2%) and is found in 10%-30% of people > 70 years of age. CHIP-related mutations (eg, DNA methyltransferase 3 alpha [ DNMT3A], tet methylcytosine dioxygenase 2 [ TET2], ASXL transcriptional regulator 1 [ ASXL1], splicing factor 3b subunit 1 [ SF3B1]) are associated with increased inflammation and elevated risk of developing hematologic malignancies, including myelodysplastic syndromes (MDS)/acute myeloid leukemia, and are an independent risk factor for cardiovascular complications. Recently, patients with MDS were reported to have high rates of cardiovascular co-morbidities including elevated levels of N-terminal pro-brain natriuretic peptide (NT-proBNP), a marker of cardiovascular and all-cause mortality. Currently, no treatments targeting CHIP mutations or associated complications are available. Luspatercept is currently approved for anemia in adult patients with lower-risk MDS (LR-MDS) with ring sideroblasts who require red blood cell (RBC) transfusions (≥ 2 RBC units/8 weeks) after erythropoiesis-stimulating agent (ESA) treatment failure. Recently, the phase 3 COMMANDS trial (NCT03682536) reported superiority of luspatercept over ESAs in reducing transfusion burden in ESA-naive patients with LR-MDS.
Aims: We investigated the association between the most frequent CHIP-related mutations ( DNMT3A, TET2, ASXL1, SF3B1) and clinical outcomes in patients treated with luspatercept from the COMMANDS trial.
Methods: Genomic DNA was isolated from bone marrow (BM) mononuclear cells, and 36 myeloid-specific somatic gene mutations were identified by targeted next-generation sequencing (400X; sensitivity 3%; Munich Leukemia Laboratory information system) at baseline. Hematologic parameters (eg, hemoglobin [Hb], complete blood counts), transcriptome data (bulk BM RNA sequencing [RNA-Seq] data) and cytokines/chemokines (peripheral blood) were assessed at baseline and week 24.
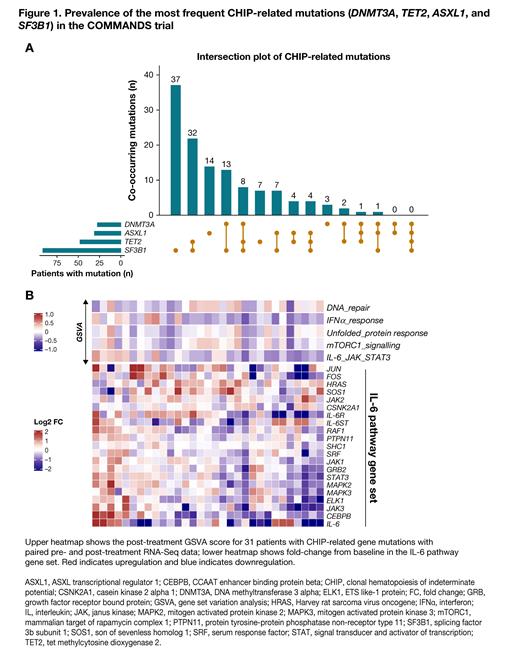
Results: Overall, 145 patients in the luspatercept arm of the COMMANDS trial were included in the current analyses, of whom 85% (123/145) (Figure 1A) had the most frequent CHIP-related mutations (eg, DNMT3A, TET2, ASXL1, and SF3B1) with a VAF of 3%-50%. Modest positive correlations were observed between DNMT3A, TET2 and SF3B1 baseline VAF and monocyte percentage ( P = 0.024, P = 0.015, and P = 0.005, respectively). Baseline Hb levels were lower in patients with frequent CHIP-related mutations than in those with less frequent/other somatic mutations. Luspatercept treatment significantly reduced anemia from baseline in patients with CHIP-related mutations (primary endpoint response 62%; n/N = 76/123). Concomitant with the Hb increase (∆ = +1.0 mg/dL; P < 0.001), we observed improvements in other cell counts, including white blood cell counts (∆ = +0.7 × 10 9 cells/L; P = 0.01). In addition, luspatercept treatment in patients with CHIP-related mutations was associated with downregulation of inflammatory gene signatures (Figure 1B) and proinflammatory cytokines/chemokines, including hepcidin (∆ = −43.9 ng/mL; P < 0.001). Furthermore, we observed upregulation of an anti-inflammatory regulator (growth/differentiation factor 15) following treatment. Most importantly, significant downregulation of NT-proBNP levels (∆ = −330 pg/mL; P < 0.05) was observed in patients with LR-MDS who received luspatercept.
Conclusions: This retrospective subgroup analysis of patients in the COMMANDS trial with the most frequent CHIP-related mutations ( DNMT3A, TET2, ASXL1 and SF3B1) demonstrated novel effects of luspatercept in clonal hematopoiesis-related consequences including improvements in anemia, cytopenias, and reduced inflammation. Most importantly, NT-proBNP and elevated hepcidin levels were significantly downregulated in responders following luspatercept treatment. These results warrant evaluation of luspatercept in patients with high-risk CHIP mutations including clonal cytopenia of undetermined significance and patients with anemia of inflammation.
Disclosures
Hasan:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Vodala:Bristol Myers Squibb: Current Employment; Mabgenex: Membership on an entity's Board of Directors or advisory committees. Hayati:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Garcia-Manero:Bristol Myers Squibb: Other: Medical writing support, Research Funding; Genentech: Research Funding; AbbVie: Research Funding. Gandhi:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Suragani:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal